SD Biosensor STANDARD Q COVID-19 Ag Test Kit (Nasal) - Box of 25 Tests
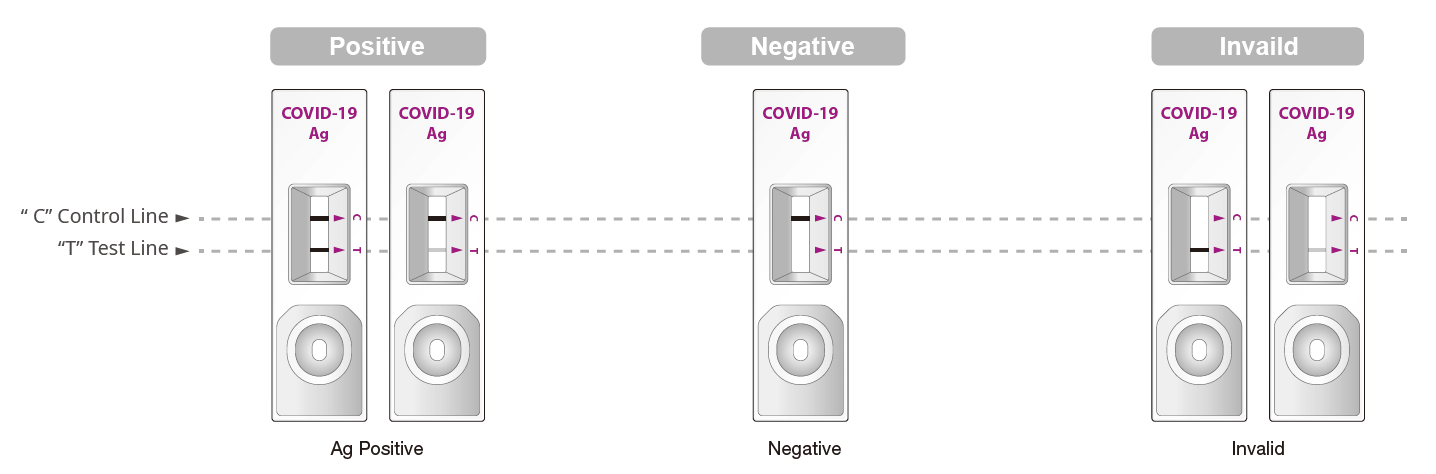
STANDARD Q COVID-19 Ag Test is a rapid chromatographic immunoassay for the qualitative detection of nucleocapsid antigens to SARS-CoV-2 present in human nasal or nasopharyngeal specimens.
Intended Use:
STANDARD Q COVID-19 Ag Test is a rapid chromatographic immunoassay for the qualitative detection of nucleocapsid antigens to SARS-CoV-2 present in human nasal or nasopharyngeal specimens. This test is for administration by healthcare workers and labs only, as an aid to early diagnosis of SARS-CoV-2 infection in patient with clinical symptoms with SARS-CoV-2 infection. It provides only an initial screening test result.
- Fast results within 15 mins
- Easy and convenient testing process
- Preferred by healthcare professionals and laboratories
- Effective in detecting the SARS-CoV-2 variant
- Specimen : Nasal & Nasopharyngeal
- Specimen volume: 3 drops
- Sensitivity: 97.12%
- Specificity 100%
- All necessary reagents provided & no extra equipment needed
- 25 tests per box
Made in Korea
WHO EUL ✔
KOREA MFDS Approved ✔
TGA Approved ✔
View PDF with full details and instructions for use
Specifications:
| Specimen | Nasopharyngeal |
| Test time | within 15 mins |
| Specimen volume | 3 drop |
| Sensitivity | Ct ≦ 25 : 97.14%(68/70), Ct ≦ 33 : 90.71%(127/140, overall : 84.97%(130/153) |
| Specificity | 98.94% (1490/1506) |
| Storage temperature | 2-30℃ / 36-86℉ |
Intended Use:
SD Biosensor STANDARD Q COVID-19 Ag Test is a rapid chromatographic immunoassay for the qualitative detection of specific antigens to SARS-CoV-2 present in human nasopharynx. This test is for administration by healthcare workers and labs only, as an aid to early diagnosis of SARS-CoV-2 infection in patient with clinical symptoms with SARS-CoV-2 infection. It provides only an initial screening test result. This product is strictly for medical professional use only and not intended for personal use. The administration of the test and the interpretation of the results should be done by a trained health professional. The result of this test should not be the sole basis for the diagnosis; confirmatory testing is required.
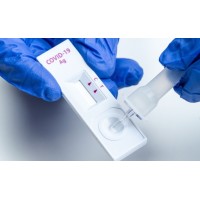
Directions for Use:
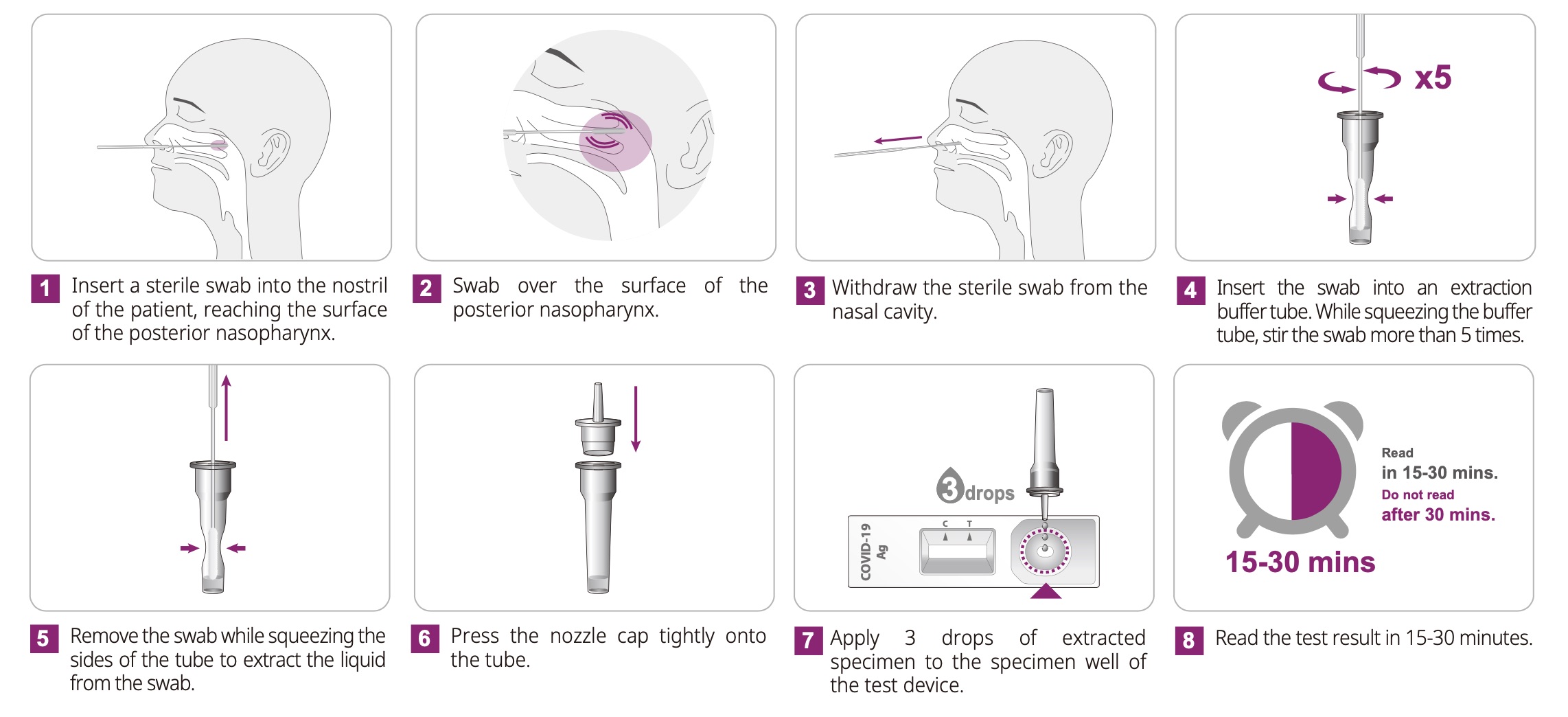
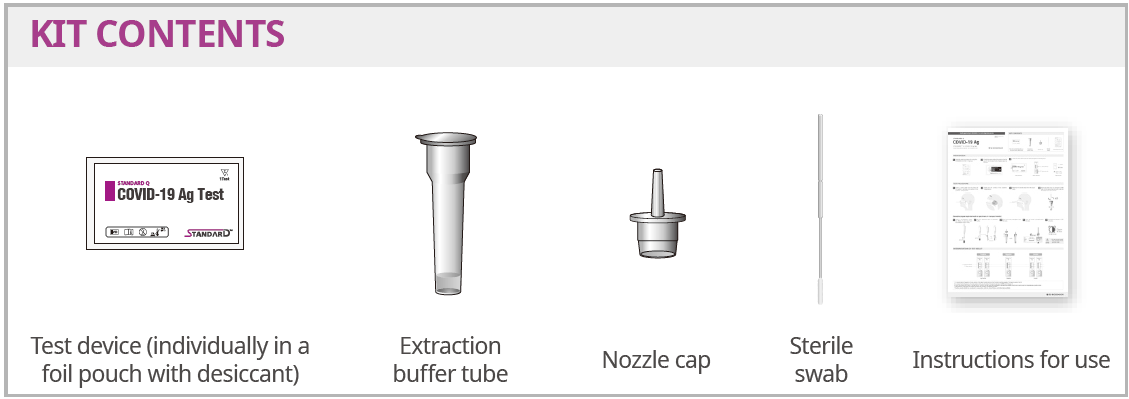
Kit Contents:
- Test device (individually in a foil pouch with desiccant
- Extraction buffer tube
- Nozzle cap
- Sterile swab
- Instructions for use
Kit Storage and Stability:
- Store the kit at 2-30°C / 36-86°F out of direct sunlight.
- Kit materials are stable until the expiration date printed on the outer box.
- Do not freeze the kit.
Product Category No. 09COV30D